GBA Pharma GmbH
Booth number: B03:40
www.gba-group.com/en/pharma/gmp-testing/
About us
GBA Pharma provides Analytical Services to pharmaceutical, biopharmaceutical, and research organizations, supporting domestic and international clients with scientifically validated and regulatory-compliant solutions. Our GMP-certified and FDA-inspected laboratories in Germany deliver expertise for product development, approval, and quality control across the entire lifecycle. With more than 170 experts and 7,500 m² of laboratory space, we combine decades of pharmaceutical analysis experience with reliable cooperation, serving a global customer base. Our certifications include EU-GMP, US(FDA)-GMP, and licenses under German and EU pharmaceutical law, ensuring strict compliance. We specialize in testing drugs, biotherapeutics, medical devices, raw materials, excipients, and packaging materials using HPLC, GC, mass spectrometry, microbiology testing, nitrosamines analysis, Extractables & Leachables (E&L), stability studies, dissolution, and many more. Services include testing active ingredients, excipients, finished and semi-finished products, packaging materials, and small-scale batches, along with method transfer, monitoring, and reporting. With state-of-the-art equipment and 20+ years of expertise, GBA delivers fast, reliable, and precise analytical solutions.
Address
Anna-Sigmund-Str. 7
82061 Neuried
Germany
E-mail: m.liebig@gba-pharma.com
Phone: +49 173 4053819
Internet: www.gba-group.com/en/pharma/gmp-testing/
Ernst-Abbe-Straße 40
89079 Ulm
Germany
E-mail: m.liebig@gba-pharma.com
Phone: +49 173 4053819
Internet: www.gba-group.com/en/pharma/gmp-testing/
Contact person:
Martin Liebig
Director Business Development & Sales
E-mail: m.liebig@gba-pharma.com
Phone: +49 173 4053819
Products & Services
The GBA Group runs two GMP-certified and FDA-inspected laboratory sites in Germany with more than 170 highly qualified employees and over 4,500 m² of lab space. These subsidiaries can draw upon decades of experience in pharmaceutical analysis and assisting with the pharmaceutical approval process in human and veterinary medicine. Over this time, they have been able to build up a global customer base by providing reliable and faithful cooperation. Our laboratories are certified according to EU-GMP, and US(FDA)-GMP, and we have a manufacturing license based on §13 (1) AMG (German pharmaceutical law) as well as an import license based on §72 par. 1 AMG and the EU Regulation (EC) No 726/2004. Testing of Active Ingredients and Excipients (Analytics in accordance with the methods of the relevant pharmacopeia (Ph. Eur., USP, BP, JP, CP).
Portfolio of Services:
Testing of Finished Products in the Field of Human and Veterinary Pharmaceuticals, Biotherapeutics, Medical Devices (Testing according to pharmacopoeia or customer method), Testing of Packaging Materials, Implementation of Analytical Methods including Transfer, Monitoring and Reporting, Testing of Semi-Finished Goods, Testing of Small Scale Batches and Batches from Development (from phase 1 to market).
Biochemical Analysis
Chemical-Physical and Pharmaceutical Tests
Stability and Storage Services
Method Development and Validation
Extractables & Leachables
Elemental Impurities – Screening and Risk
Assessment
N-Nitrosamines Testing
Packaging Material Testing
Protein Characterization
Dissolution Testing
Microbiology and Molecular Biology
Antibiotics Determination
Amino Acid Analysis
Bioassay
Particle Determination
DEG (Diethylene Glycol) and EG (Ethylene Glycol)
cIEF (Capillary Isoelectric Focusing)
qPCR
Regulatory Services
QP/Release Services
Stability studies
In the field of stability testing, we offer a comprehensive service for human and veterinary medicinal products as well as for medical devices.
Our service includes consulting and planning of your stability studies as well as all necessary testing and quality assessments.
Storage of your samples under the required conditions (e.g. ICH conditions and special conditions)
Stability study sample management
Perform the pre-planned analyses on stability samples as scheduled
Dissolution Test
The determination of dissolution rate is an essential part of quality control. Dissolution testing aims to determine the rate at which an active pharmaceutical ingredient (API) is released from its formulation under standardized conditions. In practice, the quantity of drug dissolved in the dissolution medium at time X after addition of the FDF or API is determined. The amount of time required to dissolve the desired amount of API can vary significantly depending on the product being tested. In the case of immediate release dosage forms, the test is usually carried out at a single point in time (usually 45 minutes or less). Multiple samples are collected in the case of extended or delayed release.
Bioassay
Since more than 15 years we offer efficacy testing of drugs under GMP quality standards at our site. The Erythropoietin (EPO) Bioassay is regularly performed following the specifications stated in the European Pharmacopeia (Ph. Eur.) For our customers we perform regular batch release and stability testing as well as (transfer) validation of customer specific methods to support you as a back-up laboratory or to evolve a specific test procedure for your product.
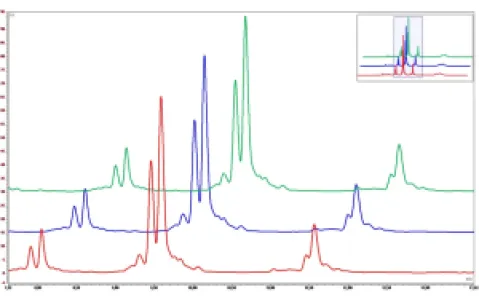
cIEF (Capillary Isoelectric Focusing)
cIEF is a technique that separates proteins based on their pI (isoelectric point), a property that reflects their overall charge at a specific pH. By employing a capillary filled with a pH gradient, proteins migrate towards their isoelectric point and separate based on their charge differences. This high-resolution separation allows for the detection of impurities, variants, or modifications in biopharmaceutical products, ensuring product purity and consistency.
Pharmacopoeial Testing – Precise, Reliable, to the Point
Pharmacopoeial methods are part of our daily routine – and it shows. Whether Ph. Eur. USP/NF, or JP: we test your products in full compliance with regulatory requirements. Our Focus? Accuracy, efficiency, and clear communication. What do we test? Everything that matters: identity, purity, content, uniformity, dissolution, and more.